Designing cells to fight cancer
How can new designs of T cells improve cell therapy for cancer patients?
This was the question Hakan Köksal attempted to answer in his PhD thesis, which he defended from the Oslo Cancer Cluster Incubator via a digital platform on Thursday 28 May 2020.
Köksal first arrived at Oslo Cancer Cluster Incubator to begin his PhD in October 2016 for the Department of Cellular Therapy, belonging to Oslo University Hospital. Three and a half years later, he is finally finished and has made a discovery that could potentially help cancer patients that are not responding to standard cell therapies.
“Essentially, what we are doing is called adoptive T cell therapy. We try to manufacture designs of chimeric antigen receptors to redirect T cells against cancer cells,” Köksal explained.
Cell therapy is an exciting, new area in cancer research and is a type of immunotherapy. This means that the patient’s immune system is changed in order to recognise and destroy the cancer cells in the body. CAR T cell therapy (CAR is short for chimeric antigen receptor) specifically involves collecting cells from the patient’s blood and changing them in the laboratory.
“We collect T cells, or lymphocytes, from the patients and engineer them so they can detect cancerous cells. Afterwards, they can be reinfused in the patient to destroy the cancer cells.” Hakan Köksal
Novel designs and new approaches
Current CAR T cell therapies have proved successful against several haematological cancers (blood cancers). However, the long-term clinical effects are quite limited and several barriers remain to cure all cancers with cell therapy. One problem Köksal looked at is when lymphoma patients treated with CD19 CAR T therapy relapse with CD19 negative lymphoma.
“We come up with alternative designs and approaches that may have an improved therapeutic effect, a lowered toxicity and improved survival in the body,” Köksal said. “The study we conducted can potentially be used as a standalone therapy or it can be complementary to reduce relapse.”
Standard CAR T therapies use antibody fragments as recognition units to detect cancer cells. In his thesis, Köksal has used a T cell receptor part, which is a different recognition domain, to increase the number of the targetable markers on cancer cells.
“Usually CAR T therapies can only detect proteins on the surface of the cell, but this new design can technically also recognise proteins inside the cell.” Hakan Köksal
Köksal stresses that we cannot know the clinical efficacy of the study before testing it in humans. The furthest they have tested is in mice, which is still a completely different organism from humans.
Read more about the research in this article: “The first Norwegian CAR”
Presenting during corona
Köksal finished his thesis in August 2019 but has not had the opportunity to defend it until now. Due to the ongoing corona situation, he could not present the trial lecture and defence in a filled auditorium but had to make do with an empty room and a laptop.
“It’s completely different. Normally, I would be standing on a stage and looking the audience in the eyes to see if I do well or bad. Now, I couldn’t see the audience, because they couldn’t share their video screens. I could only see my opponents,” Köksal explained.
In March, the corona pandemic affected the researchers in the Incubator too, because there were difficulties getting the necessary deliveries as companies worldwide had limited personnel. The laboratory had to restrict the number of people coming in and meeting rooms were temporarily converted to offices to avoid shared office space. The Incubator never closed completely and stayed open with extra sanitation procedures in place, so that the important research could go on.

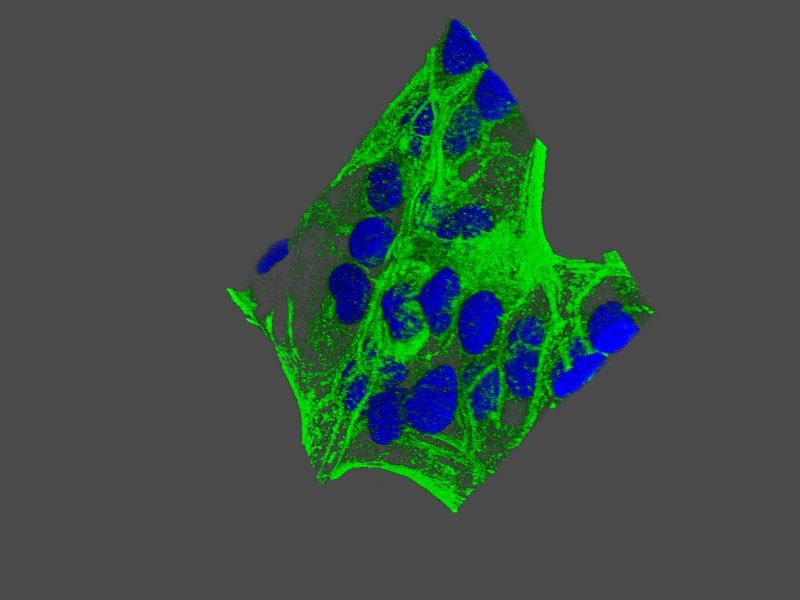
Dr. Pierre Dillard and Hakan Köksal are part of the team behind the new study on CD37CAR T-cell therapy for treatment of B-cell lymphoma.
A collaborative effort
Köksal emphasised that the research behind his PhD thesis has been a team effort. He is thankful to his supervisors at Oslo University Hospital, Else Marit Inderberg, Sebastien Wälchli and June Helene Myklebust, for helping him and giving important guidance during his projects.
It has also meant a lot for him to be a part of the Oslo Cancer Cluster Incubator, Innovation Park and the Oslo Cancer Cluster ecosystem.
“It is good to be in such a translational building. You have one part that has an arm in the clinic and at the same time you have pre-clinical research going on side-by-side with the private companies. You have different niches and you can meet a lot of people with different backgrounds and interests. It gives you new perspectives,” Köksal said.
Köksal thinks the Incubator is a calm, relaxing work environment and not super busy like many other research buildings, where there is a lot of competition going on. In the Incubator, the researchers are united by the common goal to accelerate cancer treatments.
“I feel happy when I see an announcement that a company has reached a new milestone, because it means someone is making an impact and a difference out there.” Hakan Köksal
Köksal will now begin a postdoctoral position and continue his ongoing research projects. He aims to work on the development of cell therapies and hopes to make new breakthroughs on the treatment of solid cancers in the future.